(Phosphorylation, glycosylation . ubiquitination. S-nitrosylation and Acetylation )
1.Phosphorylation-
(On serine, threonine or tyrosine residues, is one of the most important post-translational modifications involves in regulatory switches.)
Phosphorylation roles- In the regulation of many cellular processes including cell cycle, growth, apoptosis and signal transduction pathways.
2.Glycosylation-
Protein glycosylation is one of the major post-translational modifications, with significant effects on protein folding, conformation, distribution, stability and activity.
Glycosylation ouucrs in Carbohydrates in the form of aspargine-linked (N-linked) or serine/threonine-linked (O-linked) oligosaccharides are major structural components of many cell surface and secreted proteins. Makes protein hydrophillic and in acylation it makes hydrophobic.
3.Ubiquitination
Ubiquitin is a 8-kDa polypeptide consisting of 76 amino acids that is appended to the ε-NH2 of lysine in target proteins via the C-terminal glycine of ubiquitin. Polyubiquitinated proteins are recognized by the 26S proteasome that catalyzes the degradation of the ubiquitinated protein and the recycling of ubiquitin.
4.S-Nitrosylation
It reacts with free cysteine residues to form S-nitrothiols (SNOs). SNOs are often stored in membranes, vesicles, the caspases, which mediate apoptosis, are stored in the mitochondrial intermembrane space as SNOs.
5.Methylation
The transfer of one-carbon methyl groups to nitrogen or oxygen (N- and O-methylation, respectively) to amino acid side chains (lysine or arginine) increases the hydrophobicity of the protein and can neutralize a negative amino acid charge when bound to carboxylic acids. Methylation is mediated by methyltransferases, and S-adenosyl methionine (SAM) is the primary methyl group donor is acts as a co factor.
N-methylation is irreversible, O-methylation is potentially reversible. Methylation is a well-known mechanism of epigenetic regulation, as histone methylation and demethylation influences the availability of DNA for transcription. (Helps in unwinding and in reverse process ). It is common in histone 3(H3).
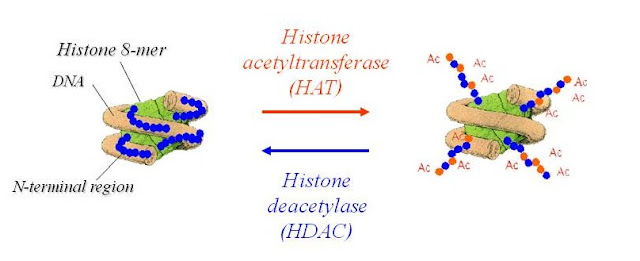
6.N-Acetylation- (unwrapping of Dna from histones)
N-acetylation, or the transfer of an acetyl group to nitrogen, occurs in almost all eukaryotic proteins. N-terminal acetylation requires the cleavage of the N-terminal methionine by methionine aminopeptidase (MAP).(Prevents degradation)
Acetylation at the ε-NH2 of lysine (termed lysine acetylation) on histone N-termini is a common method of regulating gene transcription. Histone acetylation is a reversible event that reduces chromosomal condensation to promote transcription, and the acetylation of these lysine residues is regulated by transcription factors that contain histone acetyletransferase (HAT) activity. histone deacetylase (HDAC) enzymes are co-repressors that reverse the effects of acetylation by reducing the level of lysine acetylation and increasing chromosomal condensation.
http://1.bp.blogspot.com/_ITzUKgL3xBE/SiOHw_ny6I/AAAAAAAAACo/ON2YDNj6PPQ/s1600/KIPIJ.jpg
Protein acetylation can be detected -
1.By chromosome immunoprecipitation (ChIP) using acetyllysine-specific antibodies
2. By mass spectrometry, where an increase in histone by 42 mass units represents a single acetylation.
NOTE- Protein stability is enhanced by hydroxylation of P in collagen and carboxylation of E in prothrombin.
NOTE- Protein stability is enhanced by hydroxylation of P in collagen and carboxylation of E in prothrombin.
Thank you for visiting my blog. Please feel free to share your comment on
this article. Please subscribe and share the articles to get more such articles.

No comments:
Post a Comment