Entner -Doudoroff Pathway- (By Michael Doudoroff and Nanthan Entner )
It is an alternative pathway for production of puruvate from glucose. It is different from glycolysis pathway. It uses many enzymes from both Pentose phosphate pathway and glycolysis.
It occurs in prokaryotes specially in the Gram (-)ve bacteria for example pseudomonas aeruginosa, Azotobacter,Rhizobium etc. It absent in eukaryotes.
In this pathway glucose-p is oxidised to 2-keto -3-deoxy -6-phosphogluconic acid. It is cleaved by
2-keto -3-deoxyglucose -phosphate aldolase to pyruvate and glyceraldehyde -3-phosphate .
It thrn oxidised to pyruvate by glycolytic pathway . 2 ATPs are produced by substrate level phosphorylation.
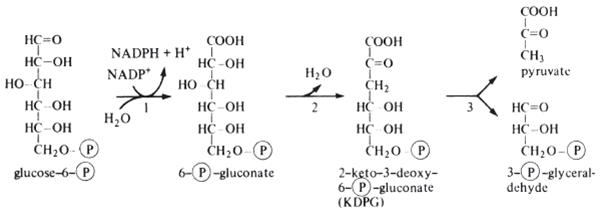
Image source -http://microbiochem.weebly.com/e
It is an alternative pathway for production of puruvate from glucose. It is different from glycolysis pathway. It uses many enzymes from both Pentose phosphate pathway and glycolysis.
It occurs in prokaryotes specially in the Gram (-)ve bacteria for example pseudomonas aeruginosa, Azotobacter,Rhizobium etc. It absent in eukaryotes.
In this pathway glucose-p is oxidised to 2-keto -3-deoxy -6-phosphogluconic acid. It is cleaved by
It thrn oxidised to pyruvate by glycolytic pathway . 2 ATPs are produced by substrate level phosphorylation.

Image source -http://microbiochem.weebly.com/e
Thank you for visiting my blog.Please feel free to share your comment on this article ,Please subscribe and share the articles to get more such articles.